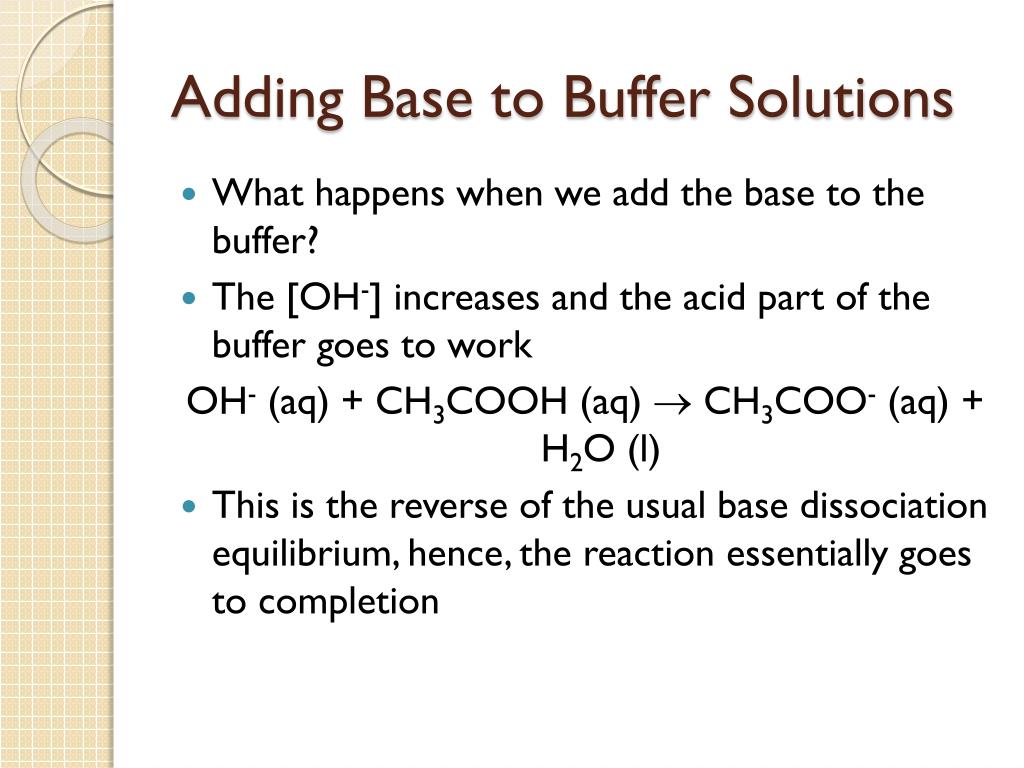
What Happens When You Add A Base To A Base . in general, a base is something that will bind tightly to a proton. if you mix equal amounts of a strong acid and a strong base, the two chemicals. there is a rule stating that we shall add a strong acid to water, and not the other way because of safety; Bicarbonate and carbonate ions are bases, and so are sulfide ions. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. now that you know \(\mathrm{hcl}\) is an acid and ammonia is a base, can you predict the reaction that occurs between them? when you add an acid or base to the buffer, the buffer components react to neutralize the added substance. This keeps the ph relatively stable. An acid and a base are like chemical. adding a base decreases the concentration of h 3 o + ions in the solution.
from www.slideserve.com
there is a rule stating that we shall add a strong acid to water, and not the other way because of safety; This keeps the ph relatively stable. An acid and a base are like chemical. adding a base decreases the concentration of h 3 o + ions in the solution. now that you know \(\mathrm{hcl}\) is an acid and ammonia is a base, can you predict the reaction that occurs between them? buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. in general, a base is something that will bind tightly to a proton. if you mix equal amounts of a strong acid and a strong base, the two chemicals. when you add an acid or base to the buffer, the buffer components react to neutralize the added substance. Bicarbonate and carbonate ions are bases, and so are sulfide ions.
PPT Chemistry 100 Chapter 17 PowerPoint Presentation, free download
What Happens When You Add A Base To A Base buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. there is a rule stating that we shall add a strong acid to water, and not the other way because of safety; This keeps the ph relatively stable. when you add an acid or base to the buffer, the buffer components react to neutralize the added substance. now that you know \(\mathrm{hcl}\) is an acid and ammonia is a base, can you predict the reaction that occurs between them? Bicarbonate and carbonate ions are bases, and so are sulfide ions. if you mix equal amounts of a strong acid and a strong base, the two chemicals. adding a base decreases the concentration of h 3 o + ions in the solution. in general, a base is something that will bind tightly to a proton. An acid and a base are like chemical. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW What Happens When You Add A Base To A Base in general, a base is something that will bind tightly to a proton. An acid and a base are like chemical. now that you know \(\mathrm{hcl}\) is an acid and ammonia is a base, can you predict the reaction that occurs between them? there is a rule stating that we shall add a strong acid to water,. What Happens When You Add A Base To A Base.
From dxolqhrho.blob.core.windows.net
What Happens When A Base Reacts With A Base at Larry Childers blog What Happens When You Add A Base To A Base in general, a base is something that will bind tightly to a proton. This keeps the ph relatively stable. Bicarbonate and carbonate ions are bases, and so are sulfide ions. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. adding a base decreases. What Happens When You Add A Base To A Base.
From studymagicmuller.z13.web.core.windows.net
The Equation Shows A Neutralization Reaction What Happens When You Add A Base To A Base when you add an acid or base to the buffer, the buffer components react to neutralize the added substance. adding a base decreases the concentration of h 3 o + ions in the solution. there is a rule stating that we shall add a strong acid to water, and not the other way because of safety; . What Happens When You Add A Base To A Base.
From www.youtube.com
ADDITION IN OTHER BASES YouTube What Happens When You Add A Base To A Base Bicarbonate and carbonate ions are bases, and so are sulfide ions. when you add an acid or base to the buffer, the buffer components react to neutralize the added substance. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. in general, a base. What Happens When You Add A Base To A Base.
From printablegragillef0.z22.web.core.windows.net
How To Combine Different Exponents What Happens When You Add A Base To A Base there is a rule stating that we shall add a strong acid to water, and not the other way because of safety; if you mix equal amounts of a strong acid and a strong base, the two chemicals. in general, a base is something that will bind tightly to a proton. Bicarbonate and carbonate ions are bases,. What Happens When You Add A Base To A Base.
From www.slideserve.com
PPT Acids and bases, salts and solutions PowerPoint Presentation What Happens When You Add A Base To A Base buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. Bicarbonate and carbonate ions are bases, and so are sulfide ions. An acid and a base are like chemical. when you add an acid or base to the buffer, the buffer components react to neutralize. What Happens When You Add A Base To A Base.
From www.youtube.com
Acid or Base in water YouTube What Happens When You Add A Base To A Base there is a rule stating that we shall add a strong acid to water, and not the other way because of safety; in general, a base is something that will bind tightly to a proton. An acid and a base are like chemical. if you mix equal amounts of a strong acid and a strong base, the. What Happens When You Add A Base To A Base.
From www.youtube.com
Base 5 Number System Addition Animated Math video elearnK12 YouTube What Happens When You Add A Base To A Base Bicarbonate and carbonate ions are bases, and so are sulfide ions. in general, a base is something that will bind tightly to a proton. there is a rule stating that we shall add a strong acid to water, and not the other way because of safety; if you mix equal amounts of a strong acid and a. What Happens When You Add A Base To A Base.
From biologysimple.com
Base Pairing Biology Simple What Happens When You Add A Base To A Base This keeps the ph relatively stable. adding a base decreases the concentration of h 3 o + ions in the solution. there is a rule stating that we shall add a strong acid to water, and not the other way because of safety; now that you know \(\mathrm{hcl}\) is an acid and ammonia is a base, can. What Happens When You Add A Base To A Base.
From www.youtube.com
Addition and Subtraction of Number Bases SHS 1 CORE MATH YouTube What Happens When You Add A Base To A Base when you add an acid or base to the buffer, the buffer components react to neutralize the added substance. An acid and a base are like chemical. now that you know \(\mathrm{hcl}\) is an acid and ammonia is a base, can you predict the reaction that occurs between them? This keeps the ph relatively stable. in general,. What Happens When You Add A Base To A Base.
From theeducationinfo.com
Acid base reaction calculator know about this calculator What Happens When You Add A Base To A Base in general, a base is something that will bind tightly to a proton. there is a rule stating that we shall add a strong acid to water, and not the other way because of safety; buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \. What Happens When You Add A Base To A Base.
From www.youtube.com
What happens to and Acid or Base in Water Solution?Acid Bases and What Happens When You Add A Base To A Base buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. in general, a base is something that will bind tightly to a proton. if you mix equal amounts of a strong acid and a strong base, the two chemicals. there is a rule. What Happens When You Add A Base To A Base.
From dxorbotyq.blob.core.windows.net
What Happens If You Add Water To A Base at Joseph Bass blog What Happens When You Add A Base To A Base in general, a base is something that will bind tightly to a proton. if you mix equal amounts of a strong acid and a strong base, the two chemicals. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. now that you know. What Happens When You Add A Base To A Base.
From support.airtable.com
How to Add a Base Collaborator Airtable Support What Happens When You Add A Base To A Base buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. An acid and a base are like chemical. now that you know \(\mathrm{hcl}\) is an acid and ammonia is a base, can you predict the reaction that occurs between them? This keeps the ph relatively. What Happens When You Add A Base To A Base.
From www.youtube.com
Mixing Strong Concentrated Acid and Base YouTube What Happens When You Add A Base To A Base in general, a base is something that will bind tightly to a proton. there is a rule stating that we shall add a strong acid to water, and not the other way because of safety; now that you know \(\mathrm{hcl}\) is an acid and ammonia is a base, can you predict the reaction that occurs between them?. What Happens When You Add A Base To A Base.
From www.youtube.com
Multiplying Exponents with the Same Base Explained! YouTube What Happens When You Add A Base To A Base An acid and a base are like chemical. there is a rule stating that we shall add a strong acid to water, and not the other way because of safety; in general, a base is something that will bind tightly to a proton. if you mix equal amounts of a strong acid and a strong base, the. What Happens When You Add A Base To A Base.
From exoxrpgge.blob.core.windows.net
Difference Between Base A And Base B Paint at Ashley Winston blog What Happens When You Add A Base To A Base An acid and a base are like chemical. Bicarbonate and carbonate ions are bases, and so are sulfide ions. in general, a base is something that will bind tightly to a proton. adding a base decreases the concentration of h 3 o + ions in the solution. buffer solutions resist a change in ph when small amounts. What Happens When You Add A Base To A Base.
From dxorbotyq.blob.core.windows.net
What Happens If You Add Water To A Base at Joseph Bass blog What Happens When You Add A Base To A Base in general, a base is something that will bind tightly to a proton. An acid and a base are like chemical. Bicarbonate and carbonate ions are bases, and so are sulfide ions. there is a rule stating that we shall add a strong acid to water, and not the other way because of safety; buffer solutions resist. What Happens When You Add A Base To A Base.